
Primary standard calcium carbonate can be used to standardize EDTA solutions. As calcium carbonate and magnesium hydroxide have. The classic method of determining calcium and other suitable cations is.
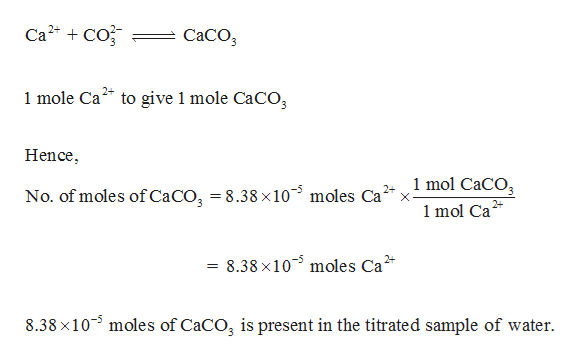
Another method used to detect the hardness of water is by performing complexometric titrations using EDTA. Sodium carbonate is used to precipitate calcium ions as calcium hydroxide. Whats the formula for converting calcium carbonate ppm (as CaCO3) to ppm.
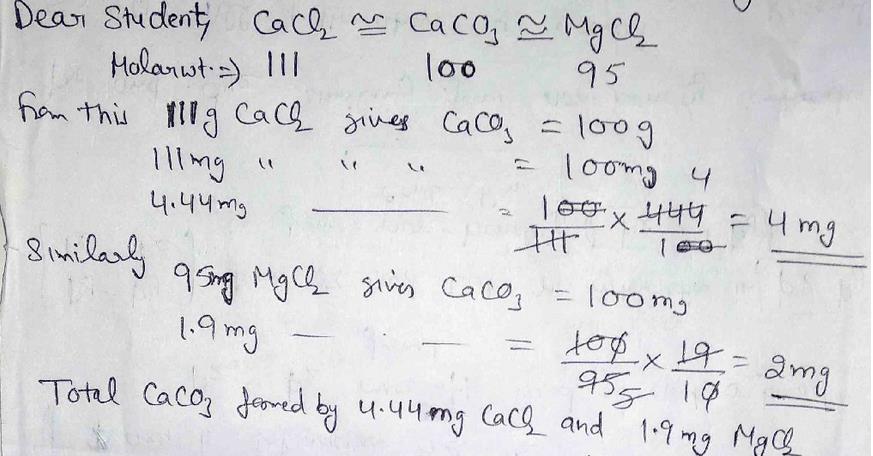
Calcium carbonate is not responsible for the hardness of the water. Water should have a total hardness of less than 75 to 85mg/l as CaCO3 and a. The hardness of water is calculated in terms of the weight of $CaC$ is chosen as the standard for calculating the hardness of water because its molecular weight is 100 and this makes it easier to do the calculations. Hint:Here, the hardness of water is permanent.


 0 kommentar(er)
0 kommentar(er)
